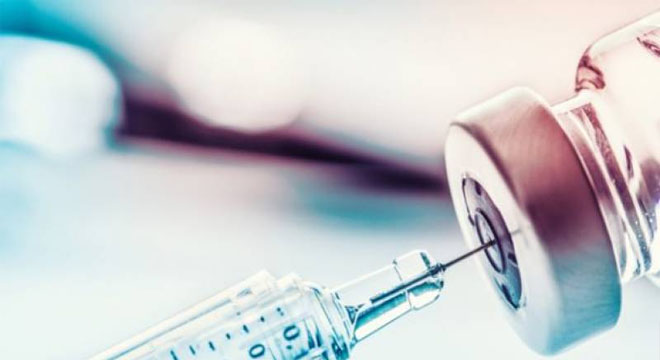
The UK has become the first country to allow the use of the Pfizer/BioNtech coronavirus vaccine on the public after the country’s regulator clinically approved the jab on Wednesday.
The vaccine, which provides 95% protection against COVID-19, will be rolled out as early as next week, according to a government statement.
“The government has today accepted the recommendation from the independent Medicines and Healthcare products Regulatory Agency (MHRA) to approve Pfizer-BioNTech’s COVID-19 vaccine for use,” it said.
“The vaccine will be made available across the UK from next week.”
The vaccine has been authorized for emergency use by the Medicines and Healthcare Products Regulatory Authority (MHRA), as its approval is still pending in the US and Europe.
“We need to all abide by these measures and we need to stick with it – let’s stick with it because help is on its way,” Health Secretary Matt Hancock said, speaking to SkyNews.
Hancock said the vaccine would be delivered by hospitals, mass vaccination centers, doctors, and pharmacies across the country, but urged the public to continue to observe rules to stem the virus’ spread.
“We can now see the way out and we can see by the spring we’re going to be through this,” he said.
“Let’s not let up and lose our resolve now – follow those rules.”
The vaccine candidate BNT162b2, developed by Turkish-German scientist Ugur Sahin’s company BioNTech, has an efficacy rate of more than 94% in adults 65 and over, according to the results of a Phase 3 clinical study.
BioNTech and Pfizer are planning to manufacture more than 50 million doses by the end of this year and potentially over 1.3 billion doses by the end of 2021.
British authorities had said they have ordered 40 million doses of the vaccine.
Follow the PNI Facebook page for the latest news and updates.