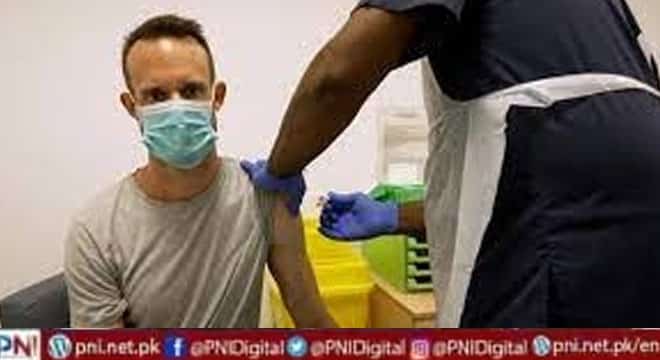
ISLAMABAD, August 30 (online) Most places in the United States with heavy monkeypox caseloads have shifted their vaccination strategies to the Biden administration’s low-dose, intradermal approach — and they are reporting varying results so far.
Some say the shift has stretched their vaccine supply, helping them meet a growing demand for the Jynneosmonkeypox vaccine. Others report a hurdle — while the new method should allow for five small doses of vaccine to be extracted from a single vial, they have only been able to extract about four.
The situation “does vary geographically,” Claire Hannan, executive director of the Association of Immunization Managers, wrote in an email to CNN.
About three-quarters of jurisdictions in the United States have shifted to using the intradermal method of administering monkeypox vaccine, Bob Fenton, the White House’s national monkeypox response coordinator, said in a briefing Friday.
“As of today, 75% of jurisdictions are already applying intradermal administration of vaccine, and another 20% are working to move in that direction,” Fenton said.
Hannan shared similar results in her email to CNN based on a recent survey of immunization managers. When asked “Has your jurisdiction implemented the intradermal vaccination strategy?” 74% of survey respondents reported yes, 3% no and 24% reported “partially.”
New US monkeypox vaccine strategy could be huge boost for supply, but much is unknown So far, the US Department of Health and Human Services’ Administration for Strategic Preparedness and Response has made a total of 1.1 million vials of Bavarian Nordic’s Jynneosmonkeypox vaccine available for free to jurisdictions to support monkeypox response efforts.
In early August, the US Food and Drug Administration issued an emergency use authorization allowing for health care providers to have the option of administering the Jynneosmonkeypox vaccine intradermally, meaning between the layers of the skin, rather than subcutaneously, or in the fatty layer below the skin, which had been the typical way the vaccine was injected.
Administering the vaccine intradermally requires a fifth of the dose needed for a subcutaneous injection, allowing providers to get as many as five doses out of a standard one-dose vial.
“It’s a little too early to tell how it’s going to help with the supply meeting demand, but I think logically, you use less vaccine for one person,” Dr. Emily Volk, president of the College of American Pathologists, told CNN.
“This is a dose-sparing approach, so it’s going to allow for the doses that we do have to be usable for many more people. So, to me that is very positive and I am actually heartened that the public health community is thinking outside the box,” she said. “It also buys time to make more vaccine.”Biden administration Friday
Without congressional funding for more kits, the government needs to conserve its stockpile in case of a fall surge of disease, the White House said.
The government will end its giveaway of Covid-19 at-home tests Friday because of insufficient congressional funding, a senior Biden administration official said Sunday.
A stockpile of the tests is being depleted, and officials want to have enough on hand in the event of a fall surge, the source said.
The giveaway, which includes tests mailed at no cost to recipients who request them at Covidtests.gov, will end Friday, according to an announcement on the site — unless there’s a surprise round of funding from Congress, the source said.
“If Congress provides funding, we will expeditiously resume distribution of free tests through covidtests.gov,” the source said. “Until then, we believe reserving the remaining tests for distribution later this year is the best course.”
Follow the PNI Facebook page for the latest news and updates.