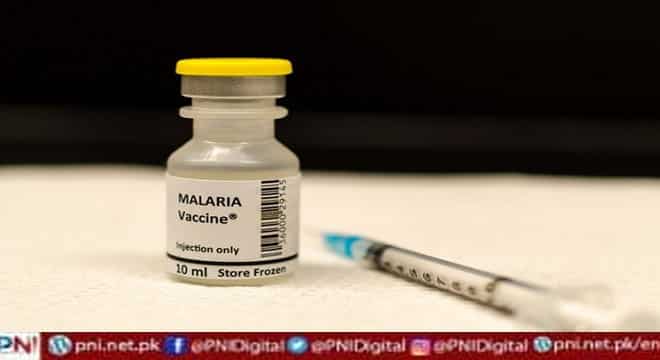
ACCRA, Apr 14 (Xinhua/APP): Ghana, one of the world’s most serious malaria-stricken countries, approved on Thursday a new vaccine for children to protect them from the deadly disease.
Delese Darko, the chief executive officer of Ghana’s Food and Drugs Authority (FDA), told a press briefing that the R21 vaccine developed by Oxford University will be used in immunizing children aged 5-36 months against malaria.
Ghana, she said, is the first country to grant market authorization for the vaccine, which is considered a major breakthrough in the fight against malaria, a common disease in West Africa.
The approval was based on a satisfactory evaluation of the quality, safety and efficacy data of the vaccine submitted to the FDA, Darko said, adding that the vaccine has a 75 percent success rate.
“The evaluation concluded that the benefits significantly outweigh the risks associated with the vaccine. Again, the vaccine has the potential to reduce the under-five mortality caused by malaria in Africa,” Darko said.
“This is a monumental event for Ghana and the rest of Africa because malaria has been a major killer disease, especially among infants on the continent. So if there is a vaccine that can protect infants against this disease, it is a refreshing development,” said Sammy Ohene, chairman of the FDA’s governing board.
According to official data from the Ghana Health Service, about 20,000 children died of malaria annually with 25 percent of them being under the age of 5.
Follow the PNI Facebook page for the latest news and updates.